
CASE NOTES
SELENIUM DEFICIENCY ON THE SOUTHERN TABLELANDS IN 2014
Alexandra Stephens, District Veterinarian Yass (South East LLS)
Posted Flock & Herd April 2015
Introduction
Clinical expression of selenium (Se) deficiency, in the form of white muscle disease in sheep and marked ill-thrift in cattle was seen in the spring of 2014 in the Yass and Monaro districts. In known selenium deficient areas, Se deficiency is expressed clinically in some years more than others. This is due to the variable uptake of selenium by plants, the variable rate of plant growth and the yearly and seasonal variance in plant species dominance. Seasons of rapid growth of shallow rooted clovers and annuals will result in less selenium being available in the plants. In 2014 the Southern Tablelands, in particular Yass and the Monaro, experienced the best autumn break in many years, followed by a mild winter and good follow up rains throughout the spring. Clover growth was at record levels and clover remained a high proportion of pasture through the winter and until mid summer.
Selenium supplementation is undertaken by some producers in a regular and structured manner by using long acting selenium supplements. The inclusion of selenium in drenches and vaccinations has meant that short acting supplements given at the right time of the year and production cycle can prevent selenium deficiency. As a result of this, less reliable autumn breaks and reduced fertilizer use selenium deficiency is now seen less often in sheep. Because supplementation becomes more ad hoc however, deficiencies may be seen in some mobs and toxicity from over supplementation in others. White muscle disease is the tip of the iceberg in a whole syndrome of clinical and subclinical disease shown to be caused by selenium deficiency.
This paper captures four of the 2014 clinical cases. It also discusses some of the difficulties in selenium deficiency detection, and discusses the seasonal variation of selenium deficiency.
Selenium availability in soils, plants and animals
Selenium content in soils varies greatly depending upon the parent rock, weathering and texture. In general, total soil Se less than 0.6 mg/kg is considered deficient.1 Soil acidity is an important factor affecting the availability of Se to plants. Selenium deficiency may be expected particularly in acid soils derived from igneous parent materials, and it may be intensified in some soils by excessive leaching, especially from irrigation. Selenium deficiency can also be seen in coastal sandy and stony soils2. In acid soils the ferric- iron selenite complex is formed, which is only slightly available to plants1. In alkaline soils most Se is present as selenates which are highly soluble and easily taken up by plants. Crops derived from Calcareous soils (eg limestone country) contain more than ten times the selenium levels of other soil types3.
When a given plant species is grown in different soils, marked differences in Se concentrations can be observed in the plant arising from differences in available Se. Concentrations in pasture of less than 0.02mgSe/kgDM result in low levels of glutathione perioxidase (GSHPx) in animals and greater than 0.05mg Se/kgDM may be adequate. Crops and pasture containing more than 0.1mg Se/kgDM will protect livestock from Se deficiency disorders1.
Heavy applications of super phosphate have been shown to decrease the concentration of selenium in pastures. Many consider that sulfate strongly inhibits the uptake of selenates by plant roots1. Others believe that it is through the rapid growth of the pasture and the change in pasture species rather than by the direct competition of sulphur with selenium for uptake4.
Rainfall received or pasture irrigation will also have a significant effect. Regions of Australia at risk of Se deficiency are those of higher winter rainfall or areas that receive more than 500mm of annual rainfall4.
Selenium is essential for animals but not for plants6. An increase in plant growth rate often leads to a decreased Se concentration in the plant, sometimes by over five-fold through a dilution of the relatively constant amount of Se available in the soil1. In grasses and clovers the highest concentration of Se is found in the leaves8. Rapidly growing pastures, particularly those that are clover dominant will be lowest in selenium availability. Slower growing and more deeply rooted plants will contain higher selenium levels. Spring growing pastures can contain half the level of selenium compared to pastures at other times of the year8.
Animal tests are the preferred method of diagnosis of selenium deficiency, rather than testing soil or plant levels. There is marked seasonal variation in the selenium nutrition of grazing livestock, the lowest levels occurring in spring and summer7. There is also considerable variation between years. White muscle disease in lambs and calves in spring is most prevalent in years when there is good autumn and winter rainfall and abundant clover growth in the spring4. In sheep, white muscle disease is observed mainly in spring born lambs on clover dominant pastures, but it is also seen in weaner sheep grazing stubble pastures. Usually only a small percentage of animals within a mob show signs of white muscle disease, usually less than 10 percent of a mob.
White muscle disease
White muscle disease is also known as nutritional muscular dystrophy (NMD). It is caused by a deficiency of selenium or vitamin E. It is seen worldwide in selenium deficient areas in horses and pigs as well as cattle, sheep and goats. White muscle disease was first described in the 1930s but it was not until 1961 that it was first shown in the USA and NZ to be a symptom of selenium deficiency. The disease is best documented in sheep. Additional factors such as stress, unaccustomed exercise and unsaturated fatty acids in pastures could contribute to disease. Clovers contain higher levels of polyunsaturated fatty acids and contain less Vitamin E than grass. The disease affects skeletal and cardiac muscle and is most common in young, rapidly growing animals, including those in utero5. White muscle disease can be prevented by Se injections to females in late gestation and/or to young stock shortly after birth1. It is thought that adequate levels of selenium protect animals from Vitamin E deficiency induced white muscle disease by not visa versa.
Difficulties with selenium measurement
Selenium deficiency also produces syndromes of ill thrift, weakness of the immune system and reproductive loss. Selenium status is measured in animals through its inclusion as an essential component of the enzyme glutathione perioxidase (GSHPx). Measurement of blood GSHPx is reasonably simple and economical, however its use does have limitations, particularly when selenium deficiency is marginal. The assays and reference values may vary between laboratories so should always be used with each lab's own reference range. We also do not seem to be able to rely upon GSHPx to accurately detect the presence of a subclinical deficiency or a selenium responsive disorder, particularly in cattle. There is considerable overlap between blood or liver glutathione perioxidase concentrations in normal animals and those suffering from both clinical and subclinical deficiency. Caution is also required in interpretation of GSHPx as it reflects selenium intake over a long period and lags at least 6 weeks (3 months) behind Se intake. Furthermore, blood and liver selenium do not provide an accurate indication of skeletal muscles levels. Selenium is associated with a large number of other proteins aside from GSHPx, some of which we do not know the function, and it is likely that they may prove in the future to have specific biochemical or metabolic functions.
When aiming to prove selenium deficiency as the cause of the problem it is important to rule out other causes of ill thrift. Dose responsive trials have been proposed as the only reliable method of diagnosing a selenium responsive condition and the only means of assessing the worth of regular supplementation in areas considered to be deficient.
When selenium supplementation is undertaken where the Se deficiency is severe, the advantages of Se supplementation can be significant and cost effective. In a New Zealand trial significant response to selenium supplementation were only seen when animals were grazing Se deficient pastures, where selenium content of pastures was less than 0.02mg/kg DM8.
Yass District cases
In mid September 2014, two separate properties were attended due to lameness progressing to weakness, collapse and death in spring born merino lambs.
Property 1 only had one mob of ewes clinically affected. These were 2 year old, maiden, fine wool merino ewes that had not received selenium in their pre-lambing drench. They were grazing on country that had not received any superphosphate for more than 15 years, but it was one of their better pastures and had grown more clover than other paddocks. The property has acidic granite based soils, and the annual rainfall for 2014 was >700mm.
Three dead lambs had been found in the paddock over the previous week. The examined lamb was able to stand but unable to walk without dragging the hind legs. It appeared to be a hind limb stiffness and paresis. There was no swelling of any of the joints, its temperature was normal, it was bright and alert with pink mucous membranes. On post-mortem examination, all of the skeletal muscles appeared normal in appearance except the hind leg long muscles; the semimembranosus, and semitendinosus. White foci of fibrosis, and areas of red inflammation were grossly evident within the muscle bellies, as was a pale discolouration of the muscles. The affected muscles were obviously different to adjoining muscle bellies.

Figure 1. White foci of fibrosis, and red inflammation grossly evident in hindlimb muscles
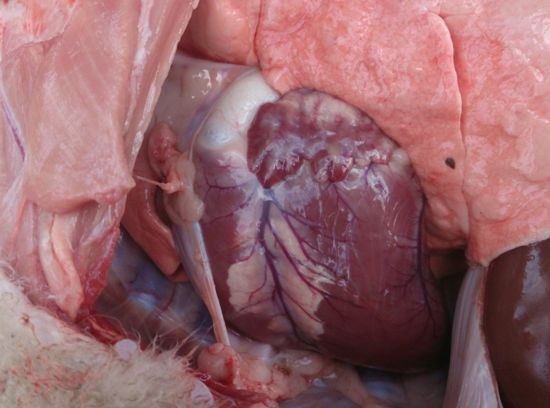
Figure 2. "Fish flesh" lesions in heart muscle
The affected lamb also had lesions on the heart. Normal heart muscle was replaced with pale "fish flesh" or white coloured muscle (Figure 2). I took photographs but I regret that the blood and histopathology samples I took were not kept as the muscle lesions were so spectacular that I believed it was unmistakably white muscle disease. Lambing had not finished and thus the owner was unwilling to muster the flock to treat all the lambs with a selenium supplement. Instead the owner opted to place multi mineral blocks that contained selenium in the paddocks and treat any affected lambs that she saw with a vaccination of 5 in 1 which contained selenium. Affected lambs were defined as 'those she could catch'. She reported that she only had 5 more cases which responded within 24 hours to treatment. Considering the considerable muscle damage evidenced on post-mortem the rapid response to Selenium supplementation is remarkable.
Property 2 had an outbreak of affected lambs in a mob of 220 maiden fine wool merino ewes. This property had a history of regular superphosphate use. In the previous 4 years in each year he had applied 100kgs/hectare single superphosphate to the paddocks. The soil type is pipe clay and soil ph (CaCl) levels generally sit around about pH 4.5. Annual rainfall for the previous 12 months had exceeded 700mm. The paddock that these sheep were in had been grazed short the previous summer and had a fantastic clover response in the autumn. The winter was mild and the clover grew unchecked into the spring. The owner estimated that the spring pasture was 80-90% clover.
The owner regularly supplements for selenium deficiency but only by using selenium in the pre-lambing drench and vaccination and by including selenium in all the drenches and vaccinations that he uses. In this case of this mob at the pre lambing treatment he had rotated to a newer drench that did not contain selenium, and coincidently had used a packet of borrowed vaccine that also did not contain selenium. This was the only mob where this had occurred.
The lambs were 1-6 weeks old (average 4 wks). The outbreak was noticed when mustering the mob to a new paddock. Six dead lambs were found in the paddock, five lambs were obviously lame and two of these could not walk. A post-mortem was performed on a small ill thrifty lamb that was able to walk. No skeletal muscle damage was seen on gross visualisation. The heart muscle was not obviously affected exteriorly but on opening the ventricles and atriums, areas of white muscle were seen on the inside of the heart. A second lamb was euthanased as it was chewing into one of its affected back legs and the muscle was secondarily infected. The condition seemed obviously painful, and the inflammation, white fibrosis and oedema were remarkable. The other hind leg was also affected with white fibrosis and inflammation of the muscle bellies of the semimembranosus, and semitendinosus.
These lambs were all treated with an injection of a short acting selenium injection in Multimin® (Virbac). Care was taken with the dosage, a vaccination syringe that could be adjusted down to deliver a dose of 0.5mls (2.5mg Se) was used. An injection of Vitamins A, D and E (25mg Vit E) was also advised and given due to the significant muscle damage seen. The owner reported very fast response to supplementation; he treated them all within 12 hours of the visit and reported that he had no further cases 24 hours after the treatment.
In less visually obvious cases of selenium responsive white muscle disease it is important to determine whether the animal has muscle damage. This can be assessed through assessing blood levels of creatinine kinase (CK), blood levels of GSHPx and histopathology.
Property 3 had a case of weaner ill-thrift in cattle which was seen in a small mob of shorthorn calves in mid October. The calves were 3-5 months old. The affected calves were obviously ill-thrifty / woody looking with very dry coats in comparison to their cohorts. This was despite a fantastic spring with pasture not limited in quality or quantity. Blood levels confirmed low levels in affected calves but levels in unaffected calves were not obtained for comparison, as they were not available for testing on the day.
| SAMPLE ID | GSHPx Units: U/gHb |
|---|---|
| 1 | 24 L |
| 2 | 19 L |
| 3 | 17 L |
| 4 | 31 L |
| 5 | 33 L |
L: low. Low normal: 40. High normal: 300units
The whole cohort of calves was treated with selenium pellets. The owner reported very good response to treatment.
MONARO CASE (Investigated by Petrea Wait, District Veterinarian Cooma)
Property 4 had a case of ill-thrift in 12-14 month old heifers. This property had a known history of Selenium deficiency seen 40 years prior as white muscle disease in lambs, and 20 years prior as ill-thrift in weaner cattle. Selcote® selenium fertilizer had been used in alternate years until about 12 years ago. The soils are acidic (soil pH CaCl around 4.7) and granite based. Superphosphate is applied regularly and annual rainfall is >750mm. The owner noted that the bottom 10% of the heifers were undersized, significantly underweight and had poor coats. They had been grazing short green pasture, but had failed to improve as the pasture availability improved and had a profuse watery to sloppy scour. They had last been drenched mid June with Nitromec® (Virbac, worm and fluke injection). The results, shown below (Tables 1-6), excluded many causes of ill-thrift but the worm count , at an average strongyle count of 232 eggs per gram was moderate. The results show exceptionally low levels of blood GSHPx and the owner reported a dramatic response to a long acting selenium injection treatment. They were also drenched at the time of sampling, so some of the response would have been in worm control but the owner felt that the most dramatic improvement was after selenium supplementation.
| SAMPLE NUMBER | RESULT | SAMPLE NUMBER | RESULT |
|---|---|---|---|
| 1 | Antibody NEGATIVE | 2 | Antibody NEGATIVE |
| 3 | Antibody NEGATIVE | 4 | Antibody NEGATIVE |
| 5 | Antibody NEGATIVE | 6 | Antibody NEGATIVE |
| 7 | Antibody NEGATIVE | 8 | Antibody NEGATIVE |
| 9 | (sample missing) | 10 | Antibody NEGATIVE |
Table 1. Pestivirus Antibody AGID test (BVD)
| SAMPLE NUMBER | RESULT | SAMPLE NUMBER | RESULT |
|---|---|---|---|
| 1 | NEGATIVE | 2 | NEGATIVE |
| 3 | NEGATIVE | 4 | NEGATIVE |
| 5 | NEGATIVE | 6 | NEGATIVE |
| 7 | NEGATIVE | 8 | NEGATIVE |
| 9 | (sample missing) | 10 | NEGATIVE |
Table 2. Pestivirus antigen capture ELISA-PACE-(BVD)-serum/skin
| SAMPLE NUMBER | LIVER FLUKE | STOMACH FLUKE |
|---|---|---|
| 1-5 | 0 | 0 |
| 6-10 | 0 | 0 |
Table 3. Worm test fluke egg count- 2 bulks, UNITS epg
| RESULT | SAMPLE NUMBER | RESULT | |
|---|---|---|---|
| 1 | <30 | 2 | <30 |
| 3 | <30 | 4 | <30 |
| 5 | <30 | 6 | <30 |
| 7 | <30 | 8 | <30 |
| 9 | <30 | 10 | 102 |
Table 4. Liver fluke ELISA on Blood samples
| SAMPLE NUMBER | STRONGYLE | NETATODIRUS | TAPEWORM | COCCIDIA |
|---|---|---|---|---|
| 1 | 160 | 0 | neg | neg |
| 2 | 420 | 0 | neg | neg |
| 3 | 380 | 0 | neg | neg |
| 4 | 120 | 0 | neg | neg |
| 5 | 20 | 0 | neg | neg |
| 6 | 220 | 0 | neg | neg |
| 7 | 120 | 0 | neg | neg |
| 8 | 440 | 0 | neg | neg |
| 9 | 400 | 0 | neg | neg |
| 10 | 140 | 0 | neg | neg |
| Average | 242 |
Table 5. Nematode egg count (modified McMaster) Units epg
| SAMPLE NUMBER | GSH PX Units: U/gHb | SAMPLE NUMBER | GSH PX Units: U/gHb |
|---|---|---|---|
| 1 | 3 L | 2 | 4 L |
| 3 | 1 L | 4 | 1 L |
| 5 | 3 L | 6 | 2 L |
| 7 | 5 L | 8 | 1 L |
| 9 | 6 L | 10 | 2 L |
Table 6. Selenium levels as indicated by blood glutathione perioxidase levels. L: low
Conclusion
Where the Se deficiency is severe, as evidenced by extremely low blood GSHPx levels, or clinical white muscle disease the advantages of Se supplementation are significant. Subclinical effects of selenium deficiency and the benefits of supplementation when selenium deficiency is marginal can be much more difficult to prove. The seasonal variation in selenium pasture levels and thus animal blood levels also makes cost effective selenium supplementation recommendations more difficult. Those animals most important to supplement are the youngest and fastest growing particularly in their first year, as well as pregnant animals, particularly those pregnant with twins. The years when it is most important to recommend supplementation are those years when pasture is clover dominated in the spring and pasture growth rates are rapid and higher than usual. Producers that use superphosphate on naturally acidic and selenium deficient soils, and have a high clover dominance of pastures and rapid growth of pasture in the spring in most years are best to use a long term selenium supplementation program. On these properties, weaners are given a long acting selenium injection at weaning and this is boosted prior to joining the following year. Alternatively slow release ruminal pellets could be used. Selcote Ultra® is a 1% selenium product that can be spread with superphosphate at 1kg/hectare to prevent selenium deficiency at the pasture level and is very effective. It was unavailable for many years but is now available again in Australia.
References
- Gupter UC & Gupta SC (2000) Selenium in Soils and Crops, Its Deficiencies in Livestock and Humans: Implications for Management Communications in Soil Science and Plant Analysis 31(11-14):1791-1807
- Parkinson TJ, Vermont JJ and Malmo J (2012) Diseases of cattle in Australasia Vet Learn pp 559-564
- Hurst R et al. (2013) Soil type influences human selenium status and underlies wide stream selenium deficiency risks in Malawi Scientific reports 3:1425
- Nutrient requirements of Domesticated Ruminants (2007) CSIRO Publishing pp152-158
- Pugh DG & Baird AN (2012) Sheep and Goat medicine 2nd edition Elsevier pp310-313
- Underwood EJ (1981) The mineral Nutrition of Livestock 2nd edition Commonwealth Agricultural Bureau, Farnham Royal, Slough, Great Britain
- Caple LW, Andrewartha KA, Edwards SJA & Halpin CG (1980) An examination of the selenium nutrition of sheep in Victoria Australian Veterinary Journal 56:160-167
- Grant AB & Shepperd AD (1983) Selenium in New Zealand pastures New Zealand Veterinary Journal 31:131-6